Service Items
Committed to creating value for clients and society in the medical sterilization industry.
About us
Be a professional contract sterilizer.

Shenzhen King Medical Packaging SterilIzation Service Co., Ltd.
King Sterilization belongs to the brand of Shenzhen King Medical Packaging Sterilization Service Co., Ltd.It is committed to providing sterilization services for sterile medical device companies and units. It is a professional service that focuses on the design and validation of sterilization of medical devices in China. Industry: Medical sterilization. King Sterilization EPCD is a registered trademark of Shenzhen King Medical Packaging Sterilization Service Co., Ltd., the trademark number is 55725495.
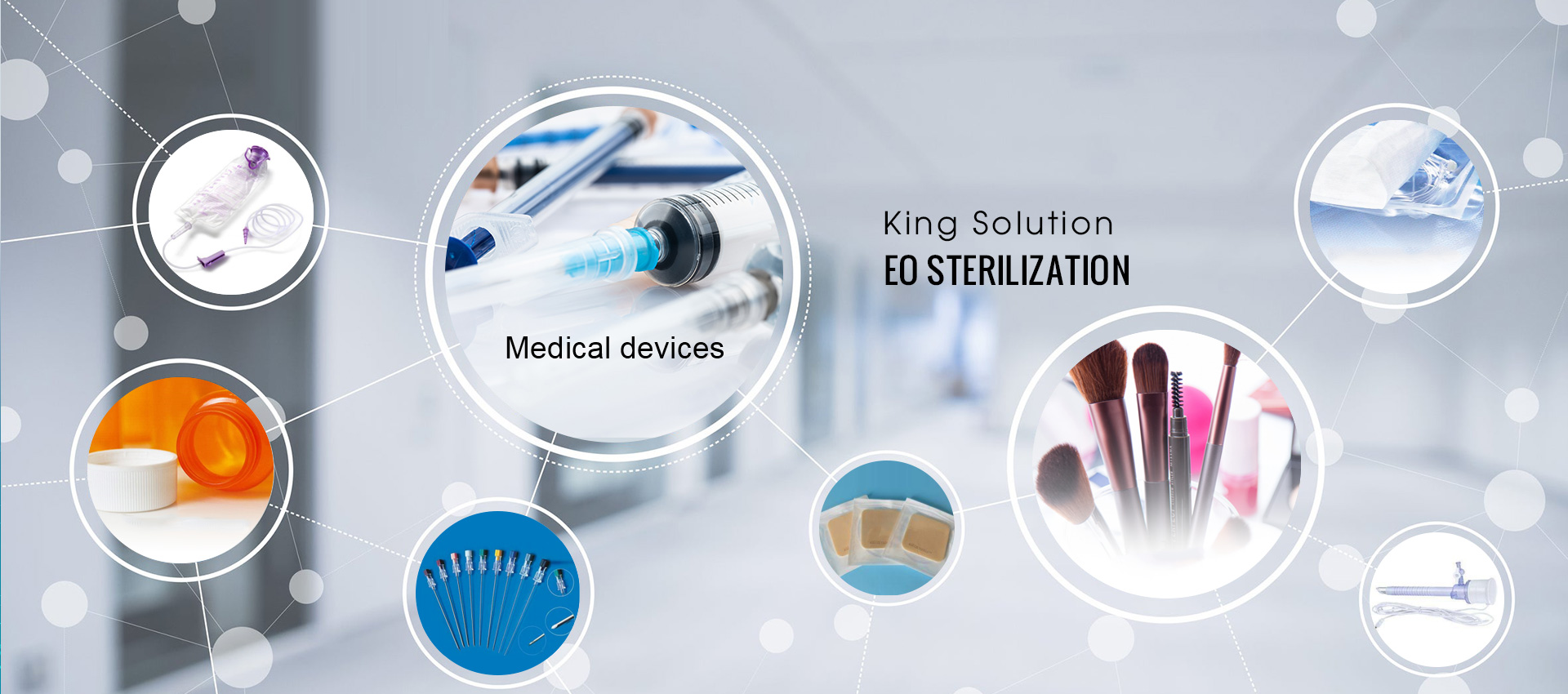
King Sterilization specializes in providing ethylene oxide sterilization processing and Sterilization validation services for medical device manufacturers, laboratory supplies, cosmetics and hygiene products and other manufacturers.......
Advantage
One-stop contract sterilizer for medical devices
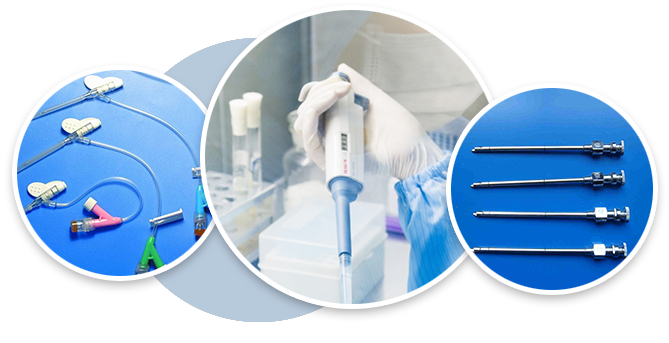
① Sterilization service · Professional external sterilization processing station
② Quality Management · Pay attention to the establishment and continuous improvement of quality management system
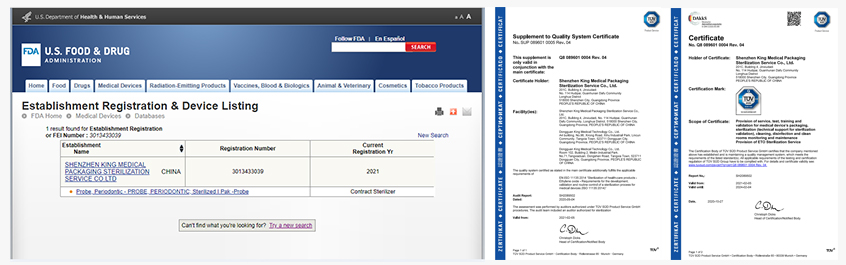
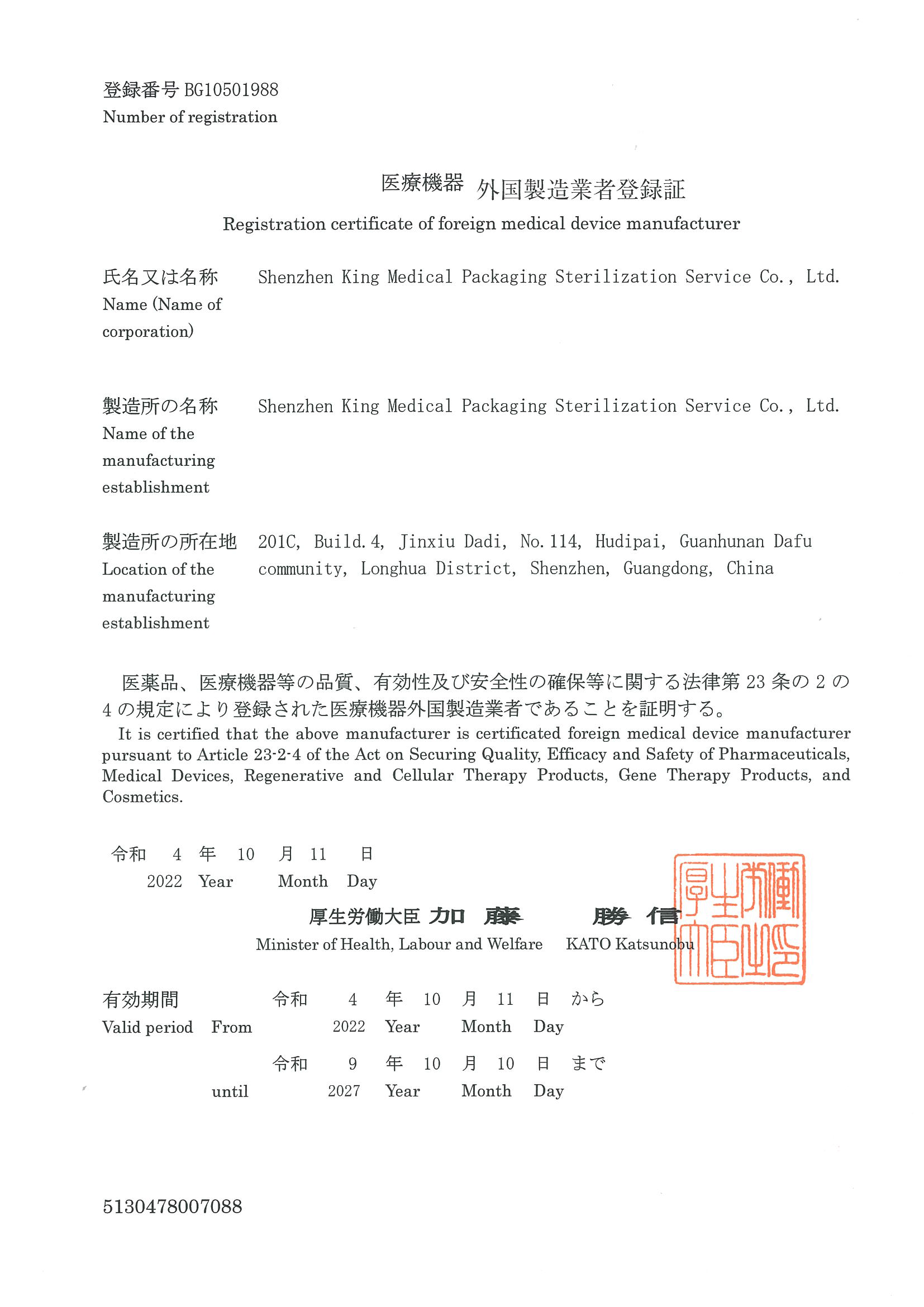
1. ISO 13485, ISO 11135 and QSR 820
2. ISO/IEC 17025 and RB/T 214
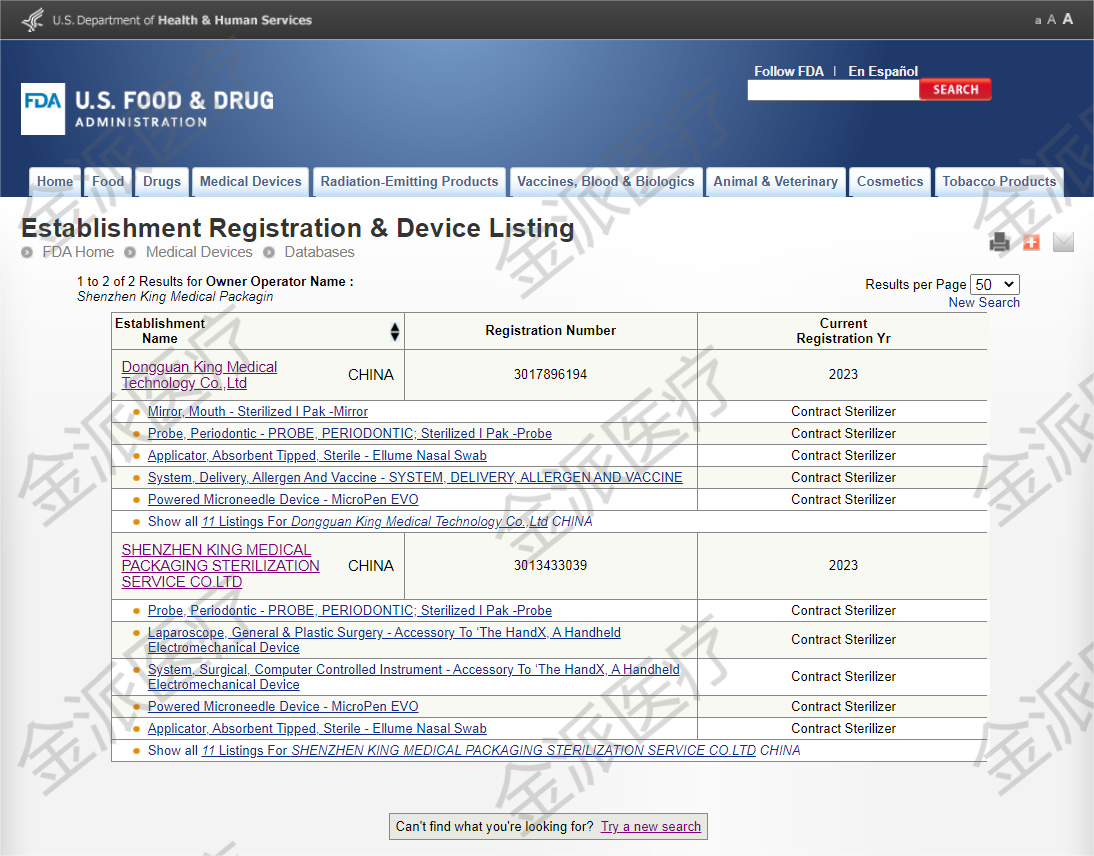
3. US FDA registration and listing

③ Professional team · Provide professional and efficient technical services for enterprises
The core members of the team have more than 15 years of work experience related to sterile medical devices, microbiology, pharmaceutical work, genetic engineering, and sterilization of sterile medical devices.
④ Sophisticated equipment· Focus on sterilization services in the medical devices’ industry
1. There are 8 sterilization chambers with different size to meet the needs of various clients
2. Daily output 200-300 cubic meters
3. The annual output is 70,000-90,000 m³ per year

Sterilization range
Provide professional ethylene oxide sterilization services for manufacturers who produce medical device, cosmetics, medical supplies and health care products.








Company dynamics
- · Special Lecture on Simulated Transportation Testing of Sterile Medical Device Packaging | Jinnan Testing | Online Training | Free Registration 2022-06-28
- · Explanation of domestic and international standards for product bioburden and sterility testing during sterilization confirmation process | Online training 2022-05-21
- · 2022 May Day Holiday Notice | Jinpai Medical 2022-04-30
- · In March 2022, Jinnan Testing, a subsidiary of Shenzhen Jinpai, will hold a theoretical and practical training course on microbiological testing of medical devices in Shenzhen! 2022-02-10
Q&A
-
Sterilization knowledge 2021-12-17
- · Principles and precautions of ethylene oxide sterilization 2021-12-17
- · How to choose suitable sterilization parameters? 2021-12-17
- · Ethylene oxide sterilization program 2021-12-17
- · Five factors affecting ethylene oxide sterilization 2021-12-17
Follow us

Scan to add WeChat friends
Fast navigation
TEL:
191 2951 0671
E-mail:
Sales01@kingservice-ps.com
ADD:
2nd Floor, No. 4, Jinxiu Science Park, No. 114, Hudi Pai, Dafu Community, Guanhunan, Longhua District, Shenzhen
Factory address:
Room 102, Building 2, Meilin Industrial Park, No. 71, Tangxia Section, Dongshen Road, Tangxia Town, Dongguan City
COPYRIGHT © SHENZHEN KING MEDICAL PACKAGING STERILIZATION SERVICE CO., LTD. ALL RIGHTS RESERVED
Powerby:www.300.cn 粤ICP备15036629号
















 0755-29362289
0755-29362289

 Messages
Messages  QQ
QQ 