Sterilization Validation Process
Daily flow chart of ethylene oxide sterilization validation :
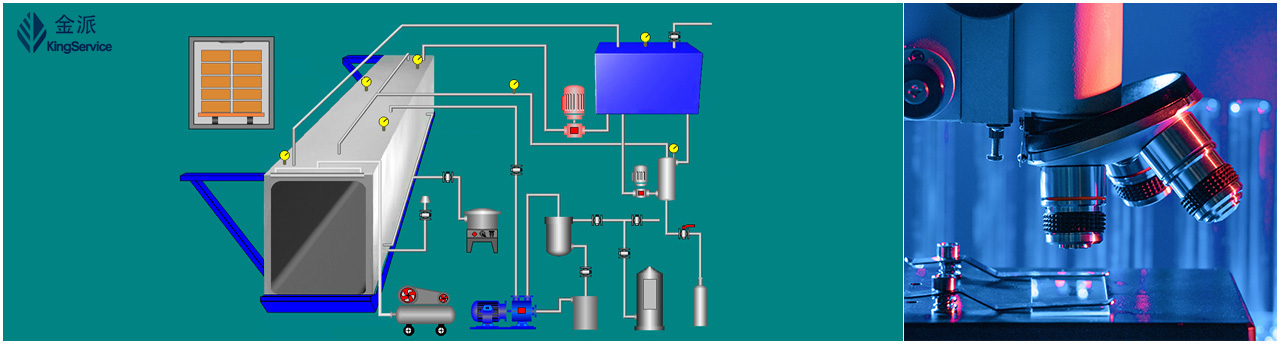
Ethylene oxide sterilization validation workflow:
| 1 | Sterilization suitability analysis of the products | Marketing department is responsible for collecting product information; |
| Technical department is responsible for sterilization suitability analysis of the products; | ||
| Marketing department makes a preliminary quotation for EO sterilization validation and routine sterilization. | ||
| 2 | Contract review and sign | Marketing department sends Technical requirement of Ethylene Oxide sterilization validation to the customer, & filled by customer. |
| Marketing department shall have the Ethylene Oxide sterilization validation contract according to Technical requirement of Ethylene Oxide sterilization validation. | ||
| Both parties shall review and sign the Ethylene Oxide sterilization validation contract. | ||
| 3 | Sterilization validation—Preparation | Marketing department sends production order of EO sterilization validation to the technical department and the laboratory. |
| Marketing department sends Preparation list for sterilization validation to the customer, and the customer prepares fillers and testing samples. | ||
| Both parties identify the most difficult location for sterilization.Technical department shall make validation protocol and get its approval from clients | ||
| Technical department shall implement sterilization validation according to the sterilization validation protocol | ||
| The laboratory implements various tests. | ||
| 4 | Sterilization validation - implementation | Technical department prepares the sterilization validation report, and provide for the customer for approval. |
| Customer approvals the sterilization validation report; | ||
| 5 | Sterilization validation - completion | Technical department shall clean the remaining dunnage and testing samples and inform the customer to take them back. |
| Technical department prepares the customer specifications for routine sterilization according to the sterilization validation report and provides for the customer under controlled documentation. | ||
| The quality department signs a quality agreement with the customer; | ||
| 6 | Routine sterilization | Marketing department signs the sterilization processing contract according to the customer sterilization requirements. |
| The production department implements EO sterilization process; | ||
| The quality department provides the batch records after sterilization |




Follow us


Scan your phone

Scan and follow the official account
Fast navigation
TEL:
191 2951 0671
E-mail:
Sales01@kingservice-ps.com
ADD:
2nd Floor, No. 4, Jinxiu Science Park, No. 114, Hudi Pai, Dafu Community, Guanhunan, Longhua District, Shenzhen
Factory address:
Room 102, Building 2, Meilin Industrial Park, No. 71, Tangxia Section, Dongshen Road, Tangxia Town, Dongguan City
COPYRIGHT © SHENZHEN KING MEDICAL PACKAGING STERILIZATION SERVICE CO., LTD. ALL RIGHTS RESERVED
Powerby:www.300.cn 粤ICP备15036629号



 0755-29362289
0755-29362289

 Messages
Messages  QQ
QQ 